PREVAIL Begins Clinical Research on Lassa Fever Study Vaccine
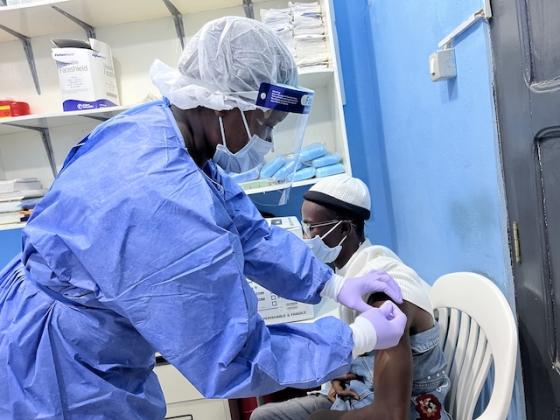
The Partnership for Research on Vaccines and Infectious Diseases in Liberia (PREVAIL), a joint Liberia-United States research partnership, has launched a Phase I clinical study to evaluate a candidate Lassa fever vaccine in adults of good health in Liberia.
The study, which is being carried out in collaboration with IAVI, a nonprofit scientific research organization and the study sponsor, is seeking to recruit 60 healthy adult volunteers at PREVAIL Redemption Hospital site in Montserrado County.
Lassa fever is a viral disease found in rats living in West Africa. Humans acquire the disease primarily through food or household items contaminated with the urine of infected rats. The disease also can be transmitted from person to person through contact with the infected person’s feces, urine, blood, or other bodily fluids.
The World Health Organization (WHO) estimates that 80% of infected individuals with the Lassa virus have no symptoms. Approximately 20% of infected individuals develop severe disease, which can affect the liver, spleen, and kidneys. There is currently no vaccine for the prevention of Lassa fever and outbreaks have occurred in several West African countries, including Nigeria, Guinea, Benin, Sierra Leone, Togo, and Liberia.
The Phase 1 study, which will provide initial information about the safety of the study vaccine and whether it stimulates potentially protective immune responses against the virus, is being conducted at three sites in the United States. Liberia is the only West African study site assessing this vaccine. The candidate vaccine was developed by IAVI, and it is similar in design to the now-licensed Ebola vaccine manufactured by Merck that was originally tested in the PREVAIL 1 study conducted in 2015 in Liberia.
PREVAIL physician Dr. Mark Kieh will lead the Lassa fever vaccine study at the Redemption Hospital which will last for 14 months. “The prospect of developing a vaccine that will prevent Lassa fever is a great step forward,” says Dr. Kieh.
“A Lassa fever vaccine would greatly reduce the disease burden by flattening the epidemic curve of seasonal outbreaks, offering hope and relief to local populations, especially rural communities who continue to suffer the brunt of this disease.”
The Lassa fever vaccine study is receiving support from the Coalition for Epidemic Preparedness Innovations (CEPI), a global alliance to finance and coordinate the development of new vaccines to prevent and contain infectious disease epidemics and pandemics.
